Tissue injuries on the giant clams are common in the wild. With their large mantle tissue mass, it is unsurprising that at least 75 predator species are known to prey on them – including nonlethal cropping of mantle tissue (Neo et al., 2015). More recently, non-invasive biopsy of mantle tissues is carried out to conduct wide-scale population connectivity studies (e.g. DeBoer et al., 2008; Neo & Todd, 2012; Neo et al., 2018; Fauvelot et al., 2019).
The video below shows how biopsy of mantle tissue is done in the field, and you can see that the giant clam immediately retracts its mantle in an attempt to protect the rest of its tissues. In the process of retracting, we sometimes tear their tissues – which can be really bad. Hence, it is important to loosen the grip once the hemostat forceps clips onto the mantle, and do a swift and quick snip to avoid damaging too much of their tissue. It does take a bit of practice to ensure that the process is non-invasive to the animal.
Does the cut wound heal?
This is a question that I often get from other researchers who asked me if the giant clam will recover from its ‘trauma’. To be frank, when I first started clipping tissue samples off giant clams for my own PhD research, I only knew that this method allowed the giant clam to stay alive with only a bit of its flesh taken. I didn’t quite know what happens after…
Fast forward a couple of years ahead, we decided to carry out an exercise to observe post-cropping of mantle tissues during mariculture operations (Neo et al., 2019). When culturing these animals, it is common practice to cut away small portions of mantle tissues and use them for inoculating symbiotic microalgae for the giant clam larvae (Ellis, 1998).
To the best of our knowledge, there has been no studies documenting wound healing abilities in the giant clams. There was a single mention by Nakayama et al. (1997) that, “The mantles which harbour symbiotic zooxanthellae must be exposed to external seawater in order to receive sunshine for photosynthesis. Since this requires keeping the shell open and exposing the giant clam to the possibility of external injury, the giant clam must possess a well-developed hemostatic system which includes the agranular cells and the morula-like cells.“

Tissues heal differently between adult and juvenile giant clams
In Neo et al. (2019), we provided a brief account of the healing and regeneration processes after the mantle edges of the fluted giant clam, Tridacna squamosa were cropped. Using photographs taken before and after the mantle tissues were wounded, the tissue recovery periods were estimated. Two types of wounding were observed in this study: artificial cropping (in a single adult clam) and cropping by three species of fish (in 20 juvenile clams).
We found that the adult clam’s mantle took ~60 days to regenerate and return to its original state but regeneration took much longer for juvenile clams (up to 90 days). Scarring occurred in the juvenile clams, but was not observed in the only adult involved in the experiment. Observations here were compared to oyster species, particularly those of high commercial importance such as Crassostrea gigas (Ruddell, 1971) and Pinctada spp. (Acosta-Salmón & Southgate, 2005, 2006). In these species, the mantle was said to completely heal and regenerated to their original extent within 90 days (Acosta-Salmón & Southgate, 2005). This corroborates with our preliminary observations.
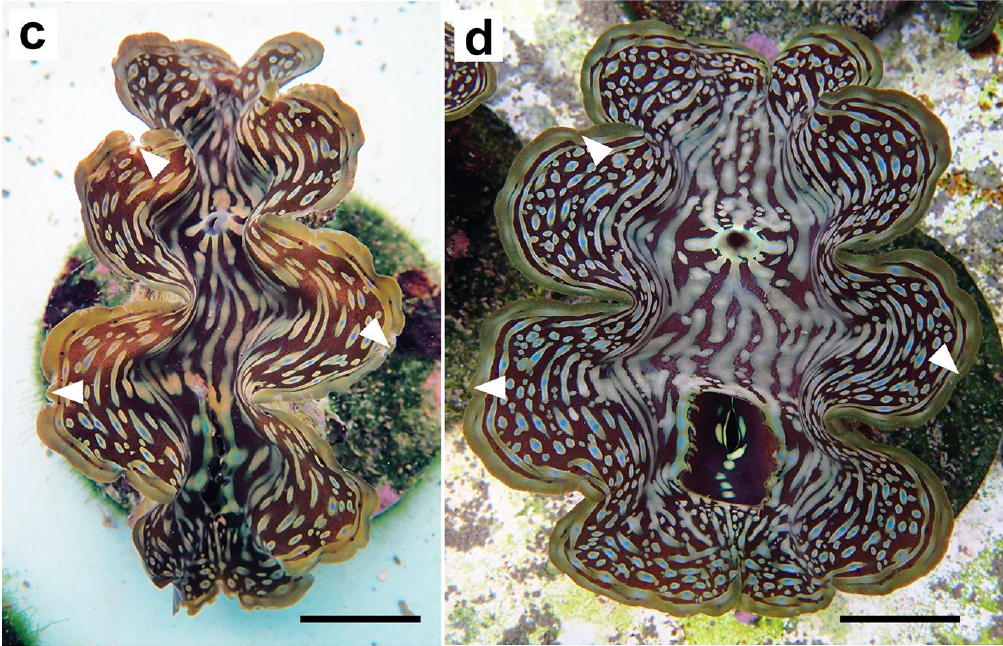
The ability of Tridacna squamosa individuals to survive and regenerate their injured tissues within three months provides preliminary insights into their responses to natural predation and can inform giant clam mariculture and restocking operations. This study is purely based on observations, and therefore, further studies are required to verify these accounts.
References cited:
- Acosta-Salmón H & PC Southgate (2005) Mantle regeneration in the pearl oysters Pinctada fucata and Pinctada margaritifera. Aquaculture 246: 447–453.
- Acosta-Salmón H & PC Southgate (2006) Wound healing after excision of mantle tissue from the Akoya pearl oyster, Pinctada fucata. Comparative Biochemistry Physiology A 143: 264–268.
- DeBoer TS, MD Subia, Ambariyanto, MV Erdmann, K Kovitvongsa & PH Barber (2008) Phylogeography and limited genetic connectivity in the endangered boring giant clam across the Coral Triangle. Conservation Biology 22: 1255–1266.
- Ellis S (1998) Spawning and Early Larval Rearing of Giant Clams (Bivalvia: Tridacnidae). Center for Tropical and Subtropical Aquaculture and Publication, Number 130, 55 pp.
- Fauvelot C, S Andréfouët, D Grulois, J Tiavouane, CCC Wabnitz, H Magalon & P Borsa (2019) Phylogeography of Noah’s giant clam. Marine Biodiversity 49: 521–526.
- Nakayama K, AM Nomoto, M Nishijima & T Maruyama (1997) Morphological and functional characterization of hemocytes in the giant clam Tridacna crocea. Journal of Invertebrate Pathology 69: 105–111.
- Neo ML & PA Todd (2012) Population density and genetic structures of the giant clams Tridacna crocea and T. squamosa on Singapore’s reefs. Aquatic Biology 14: 265–275.
- Neo ML, W Eckman, K Vicentuan-Cabaitan, SL-M Teo & PA Todd (2015) The ecological significance of giant clams in coral reef ecosystems. Biological Conservation 181: 111–123.
- Neo ML, L-L Liu, D Huang & K Soong (2018) Thriving populations with low genetic diversity in giant clam species, Tridacna maxima and T. noae, at Dongsha Atoll, South China Sea. Regional Studies in Marine Science 24: 278–287.
- Neo ML, K Vicentuan, ACF Ang & PA Todd (2019) Mantle-edge regeneration after cropping in the fluted giant clam, Tridacna squamosa Lamarck, 1819. Nature in Singapore 12: 37–42.
- Ruddell CL (1971) The fine structure of oyster agranular amebocytes from regenerating mantle wounds in the Pacific Oyster, Crassostrea gigas. Journal of Invertebrate Pathology 18: 260–268.
